Professional Medical Hip Implant Titanium Bipolar Hip Joint Prosthesis
Professional medical hip implant titanium bipolar hip joint prosthesis
What Is Hip Joint Prosthesis?
Hip Joint Prosthesis, commonly known as hip replacement surgery, is a surgical procedure to replace a damaged or diseased hip joint with an artificial prosthesis. This procedure is typically recommended for individuals with severe hip pain and limited mobility due to conditions such as osteoarthritis, rheumatoid arthritis, avascular necrosis, or hip fractures that have failed to heal properly.
Total Hip Arthroplasty (THA) is intended to provide increased patient mobility and reduce pain by replacing the damaged hip joint articulation in patients where there is evidence of sufficient sound bone to seat and support the components. THA is indicated for a severely painful and/or disabled joint from osteoarthritis, traumatic arthritis, rheumatoid arthritis or congenital hip dysplasia; avascular necrosis of the femoral head; acute traumatic fracture of the femoral head or neck; failed previous hip surgery, and certain cases of ankylosis.
Hemi-hip Arthroplasty is indicated in these conditions where there is evidence of a satisfactory natural acetabulum and sufficient femoral bone to seat and support the femoral stem. Hemi-hip arthroplasty is indicated in the following conditions: Acute fracture of the femoral head or neck that cannot be reduced and treated with internal fixation; fracture dislocation of the hip that cannot be appropriately reduced and treated with internal fixation, avascular necrosis of the femoral head; non-union of femoral neck fractures; certain high subcapital and femoral neck fractures in the elderly; degenerative arthritis involving only the femoral head in which the acetabulum does not require replacement; and patholoay involving only the femoral head/neck and/or proximal femur that can be adequately treated by hemi-hip arthroplasty

Hip Joint System Specification
| Material | Surface Coating | ||
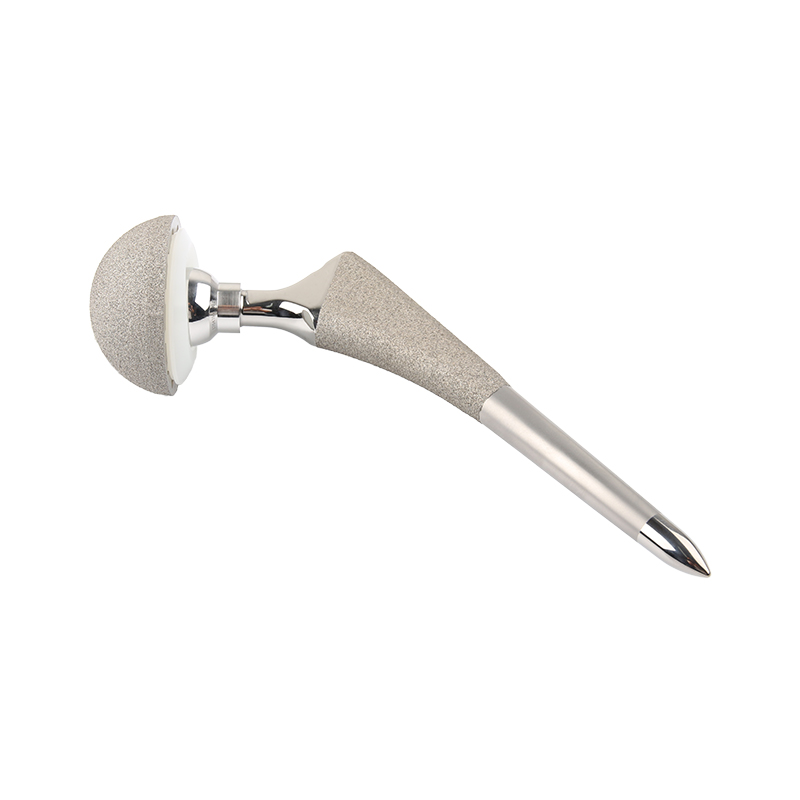
| Femoral Stem | FDS Cementless Stem | Ti Alloy | Proximal Part: Ti Powder Spray |
| ADS Cementless Stem | Ti Alloy | Ti Powder Spray | |
| JDS Cementless Stem | Ti Alloy | Ti Powder Spray | |
| TDS Cemented Stem | Ti Alloy | Mirror Polishing | |
| DDS Cementless Revision Stem | Ti Alloy | Carborundum Blasted Spray | |
| Tumor Femoral Stem (Customized) | Titanium Alloy | / | |
| Acetabular Components | ADC Acetabular Cup | Titanium | Ti Powder Coating |
| CDC Acetabular Liner | Ceramic | ||
| TDC Cemented Acetabular Cup | UHMWPE | ||
| FDAH Bipolar Acetabular Cup | Co-Cr-Mo Alloy & UHMWPE | ||
| Femoral Head | FDH Femoral Head | Co-Cr-Mo Alloy | |
| CDH Femoral Head | Ceramics |
Hip Joint Implant Introduction
Hip Joint Prosthesis Portfolio: Total Hip and Hemi Hip
Primary and Revision
Hip Joint Implant Friction Interface: Metal on highly cross-linked UHMWPE
Ceramic on highly cross-linked UHMWPE
Ceramic on ceramic
Hip Joint System Surface Treatment: Ti Plasma Spray
Sintering
HA
3D-printed trabecular bone
Hip Joint Prosthesis Femoral Stem

Titanium Hip Joint Acetabular Components

Hip Joint Implant Femoral Head

Hip Joint Replacement Indications
Intended for use in total hip arthroplasty and is intended for pressfit (uncemented) use.

Product Description




Clinical Application