Professional manufacturer PKP layer Vertebroplasty instruments set kit
Professional manufacturer PKP layer Vertebroplasty instruments set kit
Product Description
Vertebroplasty is a procedure in which a special cement is injected into a fractured vertebra with the goal of relieving your spinal pain and restoring mobility.

The Choice between PVP and PKP
PVP Preferred
1.Slight vertebral compression, vertebral endplate and backwall are intact
2.Old people, poor body condition and patients who are intolerant of long surgery
3.Elderly patients of multi-vertebral injection
4.Economic conditions are poor
PKP Preferred
1.Restoring vertebral height and correcting kyphosis is needed
2.Traumatic vertebral compressive fracture


Meet the clinical demands for both thoracic and lumbar vertebra
200psi safety margin and 300psi maximum limit
Guarantee the restoration of vertebral height and strength

Each circle 0.5ml, high accuracy of spiral propelling
On-off locking makes the operation easier.
kyphoplasty set Indications
The invalids of painful vertebral compression fractures conservative treatment in the subacute phase of osteoporotic vertebral compression fracture (<3 months)
Apparent progression of painful VCF kyphosis in the subacute phase, Cobb angle>20°
Chronic (>3 months) painful VCF with nonunion
Vertebral tumor (Painful vertebral tumor without posterior cortical defect), hemangioma, metastatic tumor, myeloma, etc.
Non-traumatic unstable spinal fracture, adjuvant treatment of posterior pedicle screw system to treat vertebral fractures, others
vertebroplasty Absolute Contraindications
● Coagulation disorders
● Asymptomatic stable fractures
● Symptoms of spinal cord compression
● Vertebral acute/chronic infection
● Allergic to bone cement and developer ingredient
vertebroplasty Relative Contraindications
● The patients of operational intolerance due to advanced age with other organ dysfunction
● The patients of VCF with facet joint dislocation or prolapsed intervertebral disc
● As the progress of surgical technique and devices, the scope of relative contraindications is narrowing as well.
Vertebroplasty kit Clinical Application



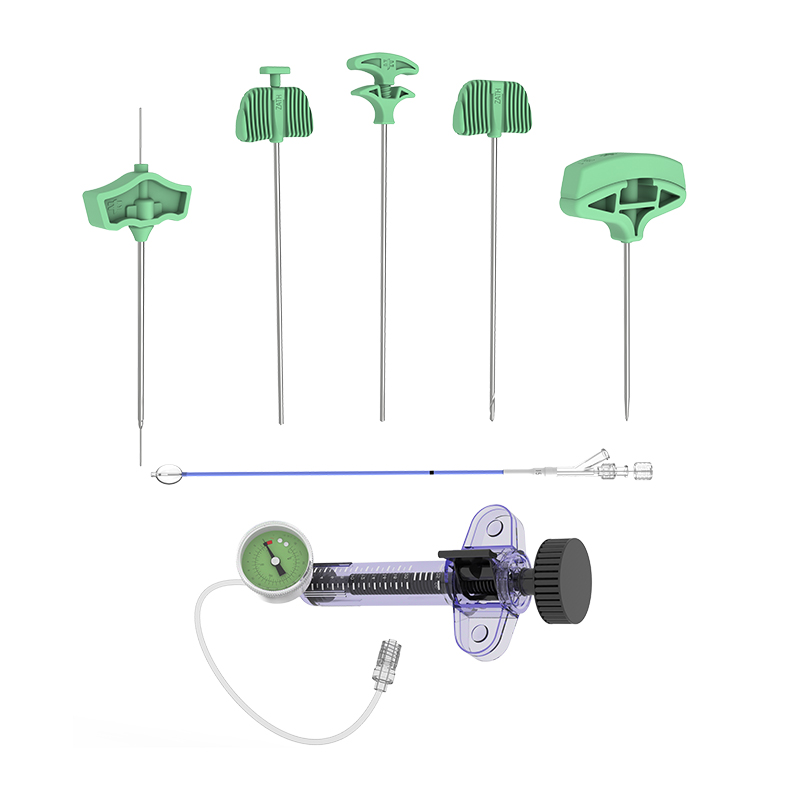
vertebroplasty set Parameter
| Puncture Needle | Φ2.5 x 130mm, Φ1.8 needle, triangular prism tip |
| Φ3.0 x 130mm, Φ1.8 needle, triangular prism tip | |
| Φ3.5 x 126mm, Φ3.0 needle, triangular prism tip | |
| Φ4.0 x 126mm, Φ3.4 needle, triangular prism tip | |
| Guiding Sleeve | Φ3.5 x 129mm, Φ3.0 needle, Φ1.5 guide wire |
| Φ4.0 x 129mm, Φ3.4 needle, Φ1.5 guide wire | |
| Guide Wire | Φ1.5, blunt tip |
| Φ1.5, triangular prism tip | |
| Drill Bit | Φ3.0 x 190mm |
| Φ3.4 x 190mm | |
| Bone Cement Filling Device | Φ3.0x195mm, Φ2.3 needle, flat tip |
| Φ3.4x195mm, Φ2.7 needle, flat tip | |
| Biopsy Extractor | Φ3.0x195mm
Φ2.3 needle, toothed tip for biospy |
| Φ3.4x195mm
Φ2.7 needle, toothed tip for biospy |
|
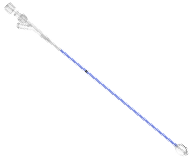
| Balloon Catheter | 10mm |
| 10mm (3.5x10) | |
| 15mm | |
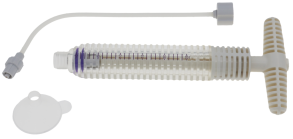
| Inflation Pump | 1~20ml/30atm |
| Bone Cement Injector | 1~30ml/30atm |
| Package | Sterile Packaging 1pcs/package |
| MOQ | 1 Pcs |
| Supply Ability | 1000+Pieces per Month |